NSE-FAST Market Research
TET Medical was interested in better understanding current diagnostic stroke protocols and refining the target product profile for their lead compound, TET-NSE-FAST. TETmedical hired Biocom Consulting that conducted a total of 7 in-depth telephone interviews with U.S. experts (4 Emergency Department Chairs and 3 Neurologists) between Jan. 27th and Mar. 9th, 2023. Interviews were 1 hour in duration and the physicians were blinded to the company and compound
The Market Research Objectives were:
- Understand current diagnostic stroke protocols and challenges
- Determine current management of stroke cases (risks, diagnostic uncertainty, etc)
- Gauge physician interest in blinded product profile and potential utility within current diagnostic protocols
Conclusions
- KOLs interviewed were enthusiastic about the NSE-FAST product profile
- Consensus that the assay was highly differentiated and would provide greater diagnostic certainty, especially in uncertain cases
- Several recommended more distinct differentiation from prior assays to avoid initial confusion
- There was significant interest in using the assay early, along with CT, in all acute stroke suspected cases
- Sensitivity and specificity of at least 90% would be well received, with even greater confidence at higher levels
- Some KOLs responded favorably to inclusion of clinically relevant data (cases missed without MRI and/or avoidance of tPA) tools provide clinical guidance but have significant limitations
- When CT imaging is negative, physicians rely heavily on degree of disabling symptoms to drive diagnosis and treatment
- Despite diagnostic advances, experts cited numerous clinical scenarios that are diagnostically challenging.
- Experts indicated 3 major areas of unmet need in ischemic stroke: greater diagnostic certainty, improved detection in difficult cases and improved pre-hospital diagnosis.
Preliminary US Stroke Opportunities
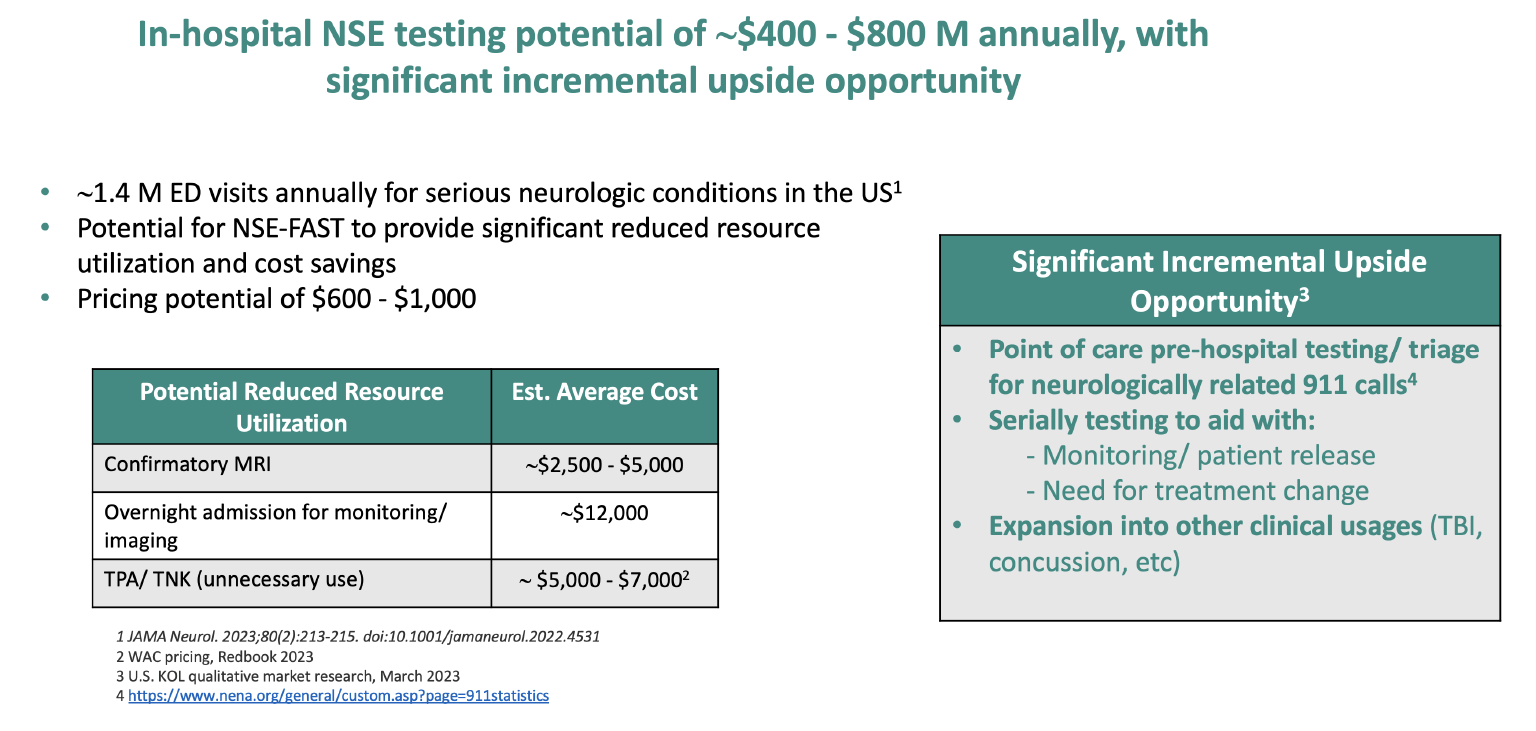
The slide below from the report describes an annual US potential market of between $400M and $800M.
Go to Market Strategy
We do not aim to change the flow and standard of care in stroke, thus assuring fast adoption. A pilot stroke study has already been conducted at Guthrie Stroke Center. A follow up feasibility study is in progress at Weill Cornell Medicine.
Our first customers will be the clinicians in the Emergency Department. The NSE-FAST will be performed on all patients presenting with a suspicion of stroke at the same time as other STAT blood work. These patients all receive a non-contrast CT scan which is used to rule out hemorrhagic stroke. Currently the clinicians have a diagnostic challenge for patients that are negative on the CT scan but symptomatic, particularly with mild or atypical symptoms. A negative TET NSE-FAST will provide high confidence to the clinician that the patient is not having an ischemic stroke; thus reducing the risk of treating a stroke mimic with tPA. We expect to initiate the training study at 1-2 clinical centers in the fall of 2023 to set the clinical cut-off followed by a pivotal study in 3-10 centers in 2024. The data from the pivotal study will be used to support the FDA regulatory pre-market submission.

