Overview
Why Is A Stroke Diagnostic So Important?
Almost half of patients going to Emergency Departments with symptoms consistent with stroke, actually have another problem that looks like a stroke, but isn’t. Of the patients with an actual stroke, ~13% have a hemorrhagic stroke, which is diagnosed via CT scan. The other 87% have an ischemic stroke, which is usually not detectable by a CT scan during the acute time period, when treatment to break up the clot provides the greatest benefits. This leaves doctors with a critical diagnostic gap: how can we very quickly distinguish patients who look like they are having a stroke from those who actually are having a stroke? Time is of the essence: every second, 30,000 neurons are lost. But treatment with a clot-busting drug (eg. Tissue plasminogen activator (tPA)) when it is not needed can put the patients at risk of actually developing hemorrhagic disease, including hemorrhagic stroke! And, treatment with that drug can cost over $6000/patient, not including the costs of hospitalization.
Acute Brain Injury IVD for Stroke
TETmedical has developed the NSE-FAST (Functional Activity Stroke Test), an In Vitro Diagnostic (IVD) for stroke to be used with standard plate readers. The test fits into standard clinical practice in the Emergency Department, with a potential high sensitivity and negative predictive value that would be highly useful with significant potential payer cost savings and reduced patient risk.
The NSE-FAST in-vitro diagnostic is in clinical studies and not yet approved by the FDA for human use.
Given the speed and specificity attainable with our diagnostic platform, TET is focused on quantitatively measuring the levels of active Neuro-Specific Enolase (NSE), a protein released by damaged neurons.
TETMedical’s Revolutionary Approach
Addressing this gap is so important that scientists and doctors have been trying for decades to identify “biomarkers,” molecules in the bloodstream that will reveal the presence of dying and damaged brain cells. Because it is found in large amounts in each neuron, the protein Neuron-specific Enolase (NSE) has been well-studied as a candidate biomarker. Unfortunately, the vast majority of these efforts tried to identify NSE by using antibodies. Antibody-based detection methods have a number of problems: they often suffer from low signal:noise ratios reducing their sensitivity, their results vary depending on the antibodies used, and they tend to take long-periods of time to perform.
Because of the speed and specificity attainable with the TET diagnostic platform, we quantitatively measure newly released NSE through its activity. Unlike antibodies, TETmedical’s NSE-FAST has intrinsic signal amplification at the earliest stage. In <2 minutes from the time that plasma is added to the wells, the TET reaction reveals the amount of recently-released active NSE. A pilot pre-clinical study in a stroke center showed a clear difference between patients who had a brain injury and those presenting with stroke mimics, all using a standard hospital-based plate reader.*
NSE-FAST Does Not Change Standard of Care Stroke Workflow
Today – patients presenting at the hospital with stroke symptom as seen in the diagram below, have a blood draw for standard blood tests than are assessed clinically based on their symptoms with an often used NIH stroke scale (NIHSS) as a quantifier for symptoms. A non-contrast CT scan is then run to identify bleeding associated with a hemorrhagic stroke. If the CT is negative (about 85% of the time) the neurologist and ER doctors must then decide if it is really a stroke from a blood clot (an ischemic stroke) or just a stroke mimic from something else. With no objected blood test for brain injury this is sometimes difficult.
Just as the diagnosis of heart attack has been revolutionized by the diagnostic assays for the biomarker High Sensitivity Troponin (current market over half a billion dollars/year), the TET NSE-FAST will revolutionize the detection of stroke. In the US today, more than 80% of patients with stroke symptoms have a negative CAT scan for hemorrhagic stroke (a bleed) and clinicians depend entirely on symptoms to make a diagnosis. Many common symptoms can be vague and unspecific to stroke. Without a biomarker to identify “real” brain injury, many patients are misdiagnosed and treated with clot busters (i.e. TPA), putting them at risk due to causing bleeds.
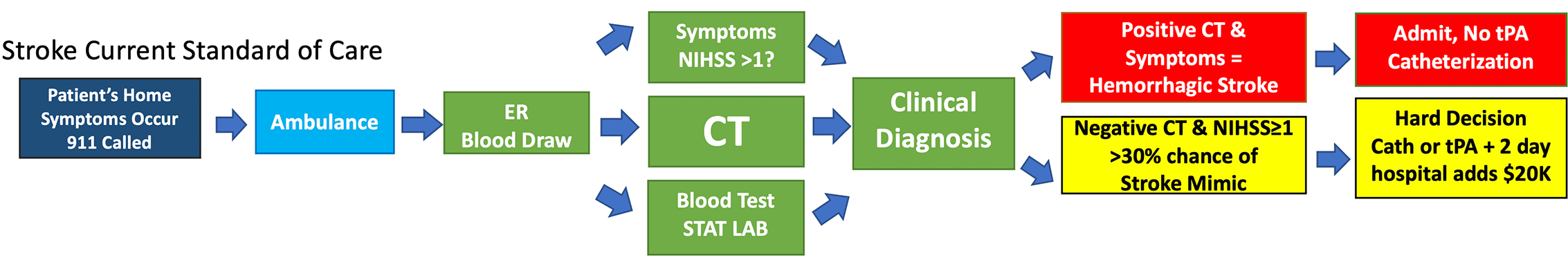
Just as the diagnosis of heart attack has been revolutionized by the diagnostic assays for the biomarker High Sensitivity Troponin (current market over half a billion dollars/year), the TET NSE-FAST will revolutionize the detection of stroke. In the US today, more than 80% of patients with stroke symptoms have a negative CAT scan for hemorrhagic stroke (a bleed) and clinicians depend entirely on symptoms to make a diagnosis. Many common symptoms can be vague and unspecific to stroke. Without a biomarker to identify “real” brain injury, many patients are misdiagnosed and treated with clot busters (i.e. TPA), putting them at risk due to causing bleeds.
About Enzymes
Enzymes are nature’s assembly line workers that as shown in the figure below, they attach to an organic compound or substrate, change it, release the result and do it again up to a thousand times a second.
How the NSE-FAST Works
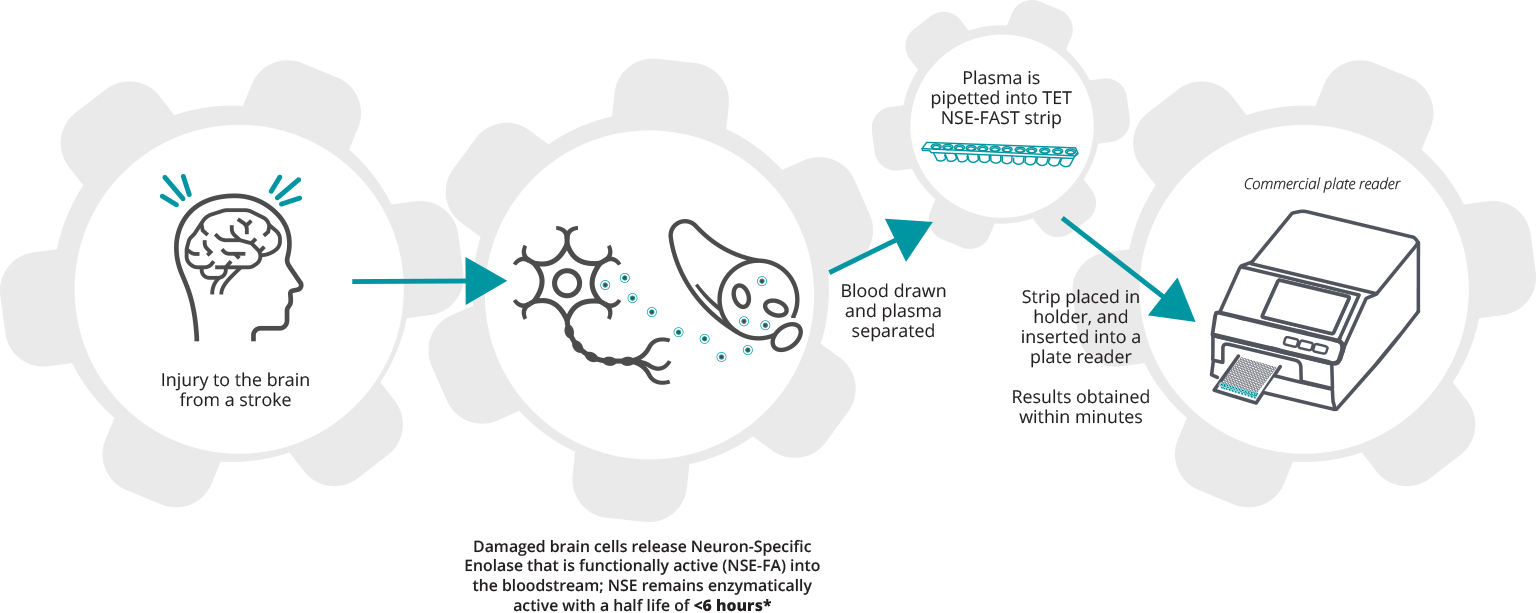
When there is injury to the brain from a stroke damaged neurons release cell contents into the bloodstream including the active enzyme NSE that makes up about 4% of the contents of a neuron. NSE remains active in the blood with a half life of <6 hours when it loses its activity but remains as a protein. Blood drawn and spun down to plasma. About 20 microliters of plasma is pipetted into each of the 12 wells in the TET NSE-FAST strip shown below. The strip that has 4 test reaction wells, 4 positive control wells and 4 negative control wells is placed in an 7 strip holder that is then inserted into a commercial plate reader that uses the luminescence from the reaction well compared to the positive and negative control wells to measure the amount of active NSE in the sample.
The NSE-FAST test reaction well contents are shown below. They include 2-PG, the substrate that the enzyme NSE takes in and changes to PEP, ADP, Luciferin and two tethered enzyme nanobots with a thousand or more enzymes tethered to a silica nanoparticle with the active side facing outward. The first nanobot has the enzyme PK that used the PEP produced by NSE to change ADP to ATP. The 2nd nanobot is Luciferase that allows fireflies to light up that takes ADP and Luciferin to emit photons.
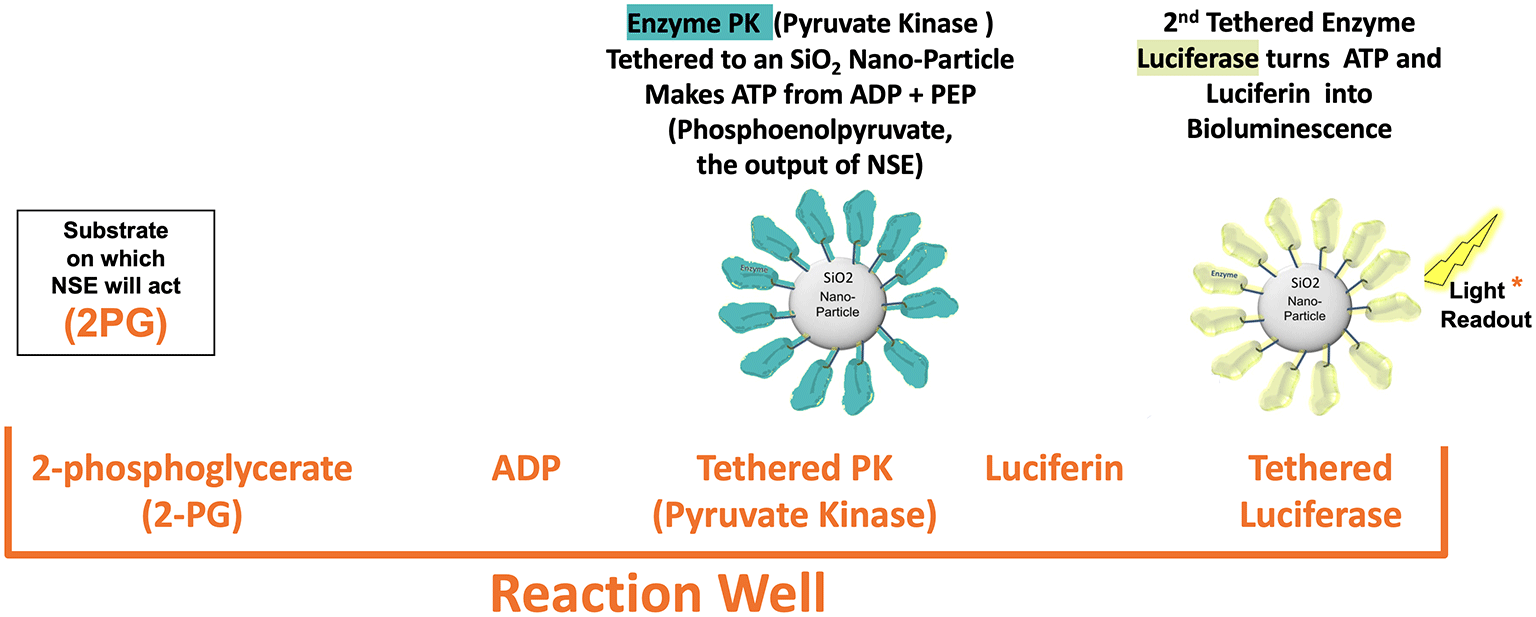
Negative control wells have everything in the reaction well above except for the 2-PG. Positive control wells include a freeze dried specific amount of enolase that will activate when plasma wets the well contents.
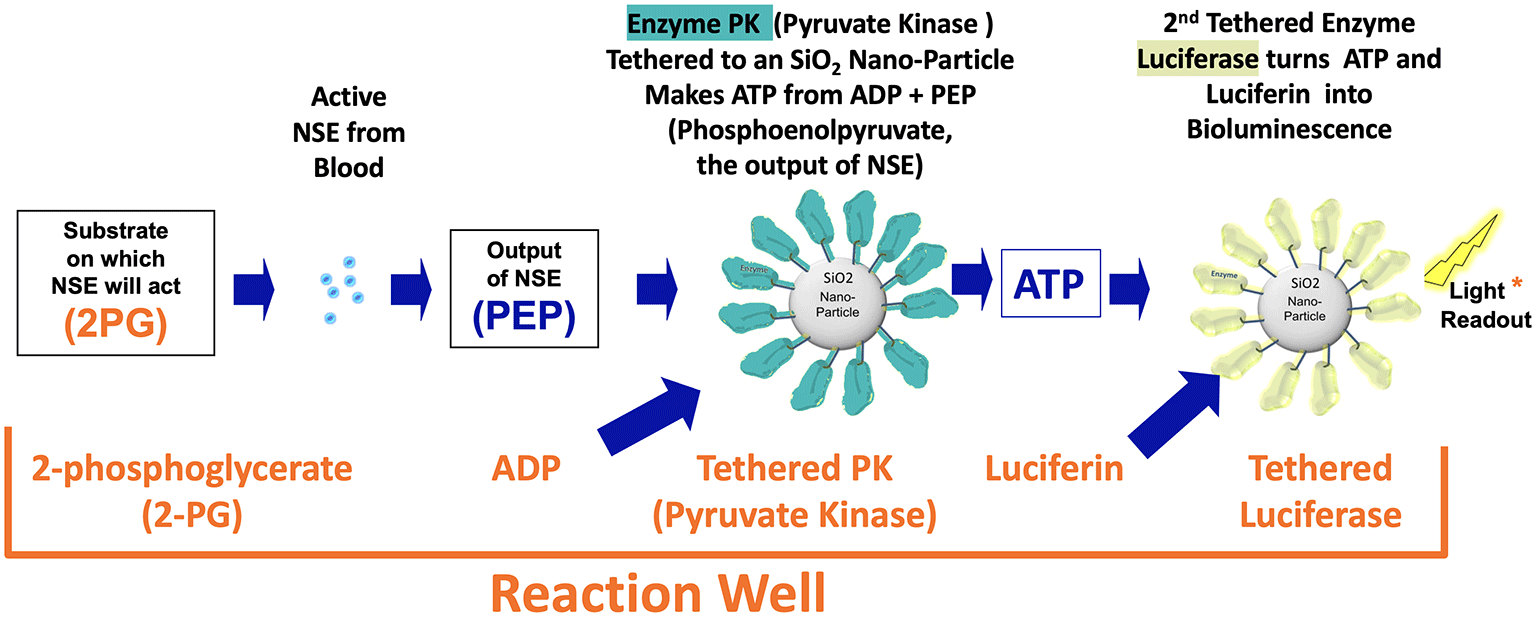
The diagram below shows the steps that occur as the catalytic reaction occurs to create luminescence when there is enzymatically active NSE in the plasma sample placed into the reaction well.
- Active NSE molecules that are in the plasma pipetted into the reaction well hydrate the freeze dried contents and begin converting 2PG to PEP at up to a thousand conversions per second per NSE molecule.
- Once the PEP is available the PK nanobots will begin converting the PEP plus ADP to ATP.
- Once the ATP is available, the luciferase nanobots will begin taking the luciferin and ATP in to emit one photon per ATP molecule
If each enzyme can do up to a thousand conversions per second than a nanobot with a thousand enzymes can do a million per second and with a thousand nanobots you get a billion per second. So with the 2PG, ADP and Luciferen saturated in the wells, the limitation on light output is only the amount of active NSE.
Product In Action
The VIDEO (to the right) shows the speed at which this assay works with first results within 30 seconds of insertion in the plate reader.
Current State Of Testing
The NSE-FAST has been tested in animals (see publications) and in two tests in humans. These include the Guthrie Pilot Study in patients presenting with stroke symptoms and a test in Mixed Martial Arts (MMA) fighters.
Descriptions of these pilots and the results obtained can be seen in the below links.
- Guthrie Pilot Stroke Study
- MMA brain injury pilot Study
*article in publication.
NSE-FAST Market Research
TET Medical was interested in better understanding current diagnostic stroke protocols and refining the target product profile for their lead compound, TET-NSE-FAST. TETmedical hired Biocom Consulting that conducted a total of 7 in-depth telephone interviews with U.S. experts (4 Emergency Department Chairs and 3 Neurologists) between Jan. 27th and Mar. 9th, 2023. Interviews were 1 hour in duration and the physicians were blinded to the company and compound
The Market Research Objectives were:
- Understand current diagnostic stroke protocols and challenges
- Determine current management of stroke cases (risks, diagnostic uncertainty, etc)
- Gauge physician interest in blinded product profile and potential utility within current diagnostic protocols
Conclusions
- KOLs interviewed were enthusiastic about the NSE-FAST product profile
- Consensus that the assay was highly differentiated and would provide greater diagnostic certainty, especially in uncertain cases
- Several recommended more distinct differentiation from prior assays to avoid initial confusion
- There was significant interest in using the assay early, along with CT, in all acute stroke suspected cases
- Sensitivity and specificity of at least 90% would be well received, with even greater confidence at higher levels
- Some KOLs responded favorably to inclusion of clinically relevant data (cases missed without MRI and/or avoidance of tPA) tools provide clinical guidance but have significant limitations
- When CT imaging is negative, physicians rely heavily on degree of disabling symptoms to drive diagnosis and treatment
- Despite diagnostic advances, experts cited numerous clinical scenarios that are diagnostically challenging.
- Experts indicated 3 major areas of unmet need in ischemic stroke: greater diagnostic certainty, improved detection in difficult cases and improved pre-hospital diagnosis.
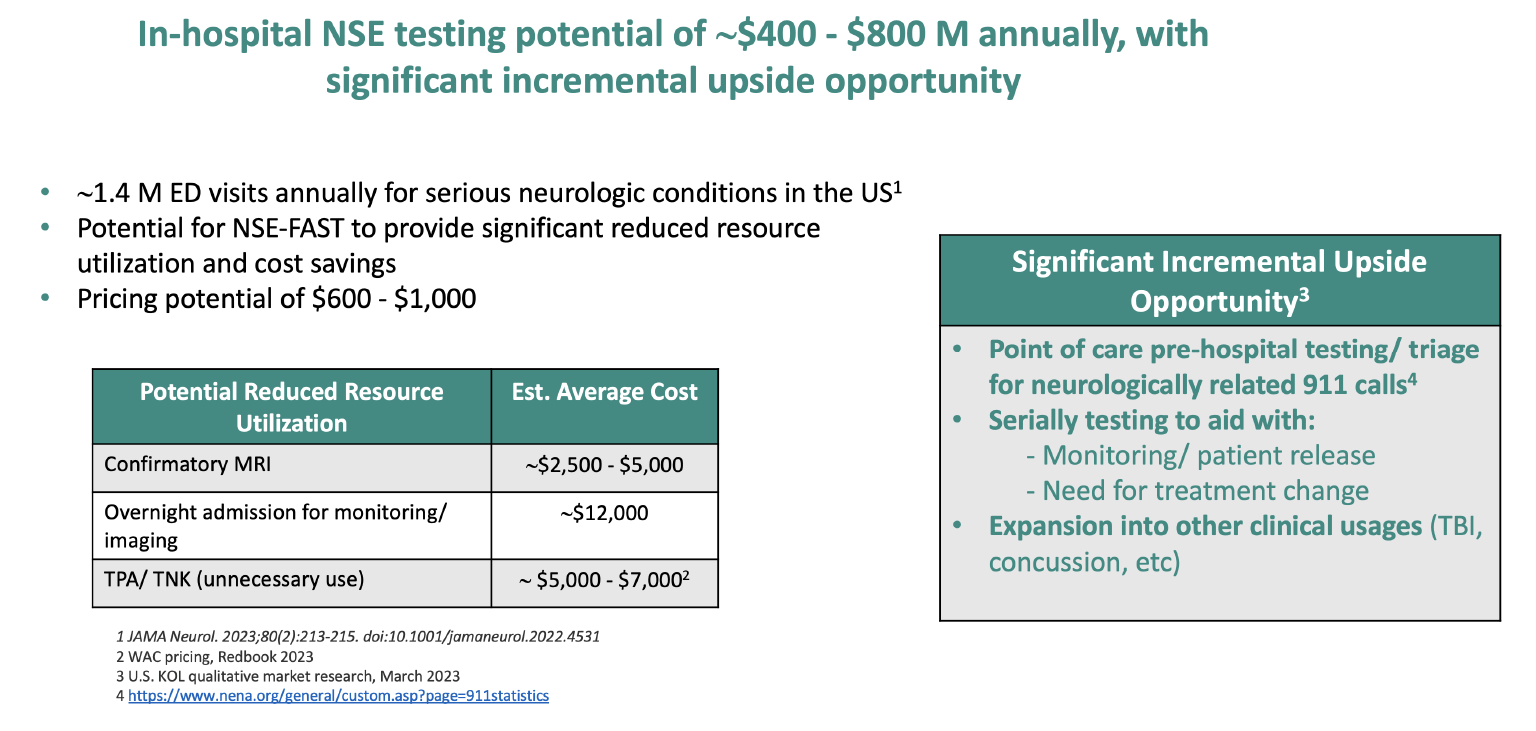
Preliminary US Stroke Opportunities
The slide below from the report describes an annual US potential market of between $400M and $800M.
Go to Market Strategy
We do not aim to change the flow and standard of care in stroke, thus assuring fast adoption. Our stroke clinical trial for FDA approval will begin in the second quarter of 2023 with sites at Guthrie Stroke Center in Northern Pennsylvania and Weill Cornell Medicine at the New York Presbyterian Hospital, the top hospital in New York City where our study has already received IRB approval without patient informed consent.
Our first customers will be Emergency Departments. There, all patients admitted with suspicion of stroke receive a CT scan – which if negative, rules out hemorrhage. TET NSE-FAST will then be performed at the same time as the regular IVD blood analysis. If negative, TET NSE-FAST provides tremendous value: to the patient (reduces risk of bleed with TPA); to the payer and hospital system (patients can be discharged, saving over $20k in TPA and admission costs; reduce litigation risk). If positive, TET NSE-A provides additional assurance that recent brain injury has occurred, helping with treatment decisions. We expect the training study in 1-2 center for the TET NSE-FAST IVD assay to begin in the fall of 2023, the pivotal study in 3-10 center to run in 2024 with FDA approval to follow.
Current State of Testing
The Neuron Specific Enolase Functional Activity Stroke Test (NSE-FAST) has been tested in animals (see publications) and in two tests in humans. These include the Guthrie Pilot Study in patients presenting with stroke symptoms and a test in Mixed Martial Arts (MMA) fighters.
Descriptions of the Guthrie Pilot Study and MMA brain injury pilot and the results obtained can be seen in below.
Guthrie Pilot Stroke Study
The TET NSE-FAST provides an objective measure of acute brain injury associated with strokes – the leading cause of disability in the US, but improperly diagnosed in the ambulance half of the time. Today, the gold standard for identifying a stroke is the NIH Stroke Score (NIHSS) based on patient symptoms only.
A pilot study in patients presenting with stroke symptoms was run at the Gutherie Stroke Center in northern Pennsylvania with results pending publication that demonstrated the utility of the NSE-FAST for detecting acute brain injury.
MMA Pilot
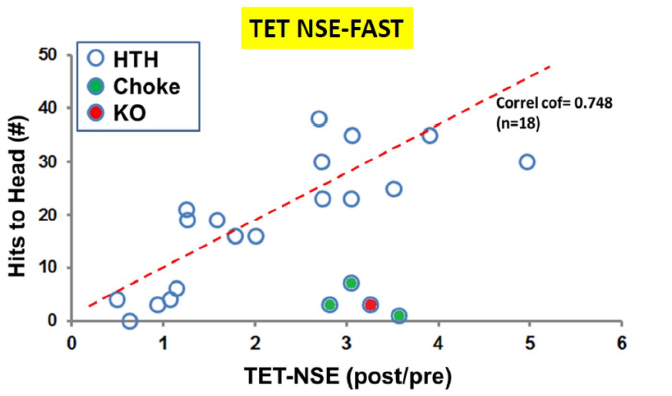
To evaluate applying TET to concussion, the TET assay was brought to a mixed martial arts tournament in Rochester, NY where fighters had blood drawn and measured for NSE activity before and after a 15 minute fight. Students in the audience counted the times each fighter was hit in the head. The plot below shows that one can correlate the number of hits to the head with the increase in NSE activity.